Requirements for Animal Testing in NZ Law
Some animals used each year for science in NZ are used because of outdated regulatory requirements.
For example, legislation and various standards require data from animal tests to try and prove the safety and efficacy of foods, chemicals and more!
Thanks to the support of LUSH Cosmetics, we were able to commission a legal review of the current requirements for animal testing in NZ law, so now we know exactly what outdated laws we need to change first!
The team of lawyers examined NZ law covering:
- Medicines and Medical Devices: Requirements for animal tests were discovered.
- Veterinary Medicine: Requirements for animal tests were discovered.
- Hazardous Substances (Including Cosmetics): Requirements for animal tests were discovered.
- Various Areas: No requirements were found in these areas.
The various areas included:
- Consumer products (general and regulated).
- Electric and gas products and appliances.
- Food and items in contact with food.
- Pet food.
- Psychoactive substances.
- Vehicles and vehicle equipment.
- Workplace products.
- Vaping products.
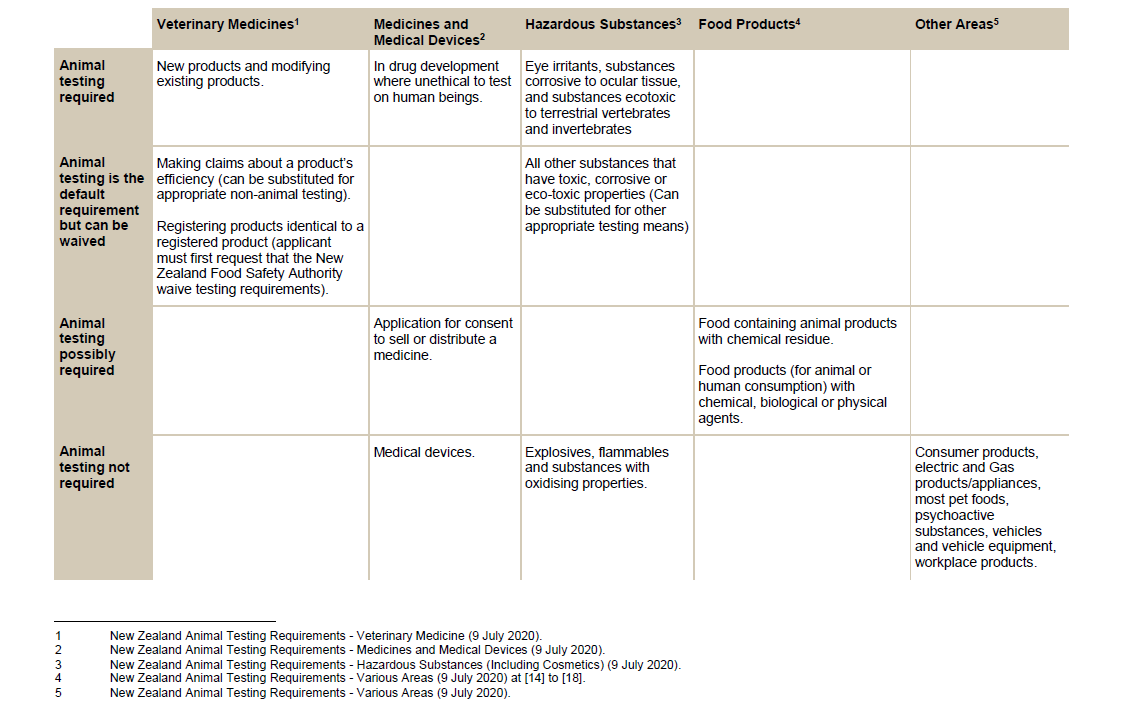
REQUIREMENTS FOR ANIMAL TESTING IN NZ LAW
Veterinary Medicines
Animal testing is required for new products and when existing products are modified.
Examples of some of the animal tests required:
- Administering a product to an animal and gathering information on the residue levels in a specified number of animals over one or multiple time points.
- Identifying any metabolites and the quantities present in different animal tissues (such as fat, muscle, kidneys) and waste matter discharged from the body (i.e. faeces and urine).
- Animal transfer studies to test for the maximum potential residue transferring into animal tissues from consumption.
Medicines and Medical Devices
Animal testing is required in drug development, where it’s unethical to test on human beings. For example, where a medicine may have genotoxic, carcinogenic, or teratogenic effects.
Hazardous Substances
Animal testing is required for: Eye irritants, substances corrosive to ocular tissue, and substances ecotoxic to terrestrial vertebrates and invertebrates.
Examples of some of the animal tests required:
- Acute oral toxicity: This test normally uses rodents. The test substance is administered in a single dose to animals fasted prior to dosing. The first animal is dosed a step below the best preliminary estimates of the median lethal dose (LD50). The second animal receives a lower dose (if the first animal dies) or a higher dose (if the first animal survives). All animals are observed and autopsied.
- Acute dermal toxicity: Groups of animals of a single-sex are exposed to the test chemical through the dermal route. Subsequently, observations of effects and deaths are made. Generally, animals that die during the test are autopsied, and at the conclusion of the test, the surviving animals are killed and autopsied.
- Acute inhalation toxicity: The preferred species for this test are rats. Animals are exposed to a pre-defined concentration of the test substance through inhalation chambers. Animals are then observed for clinical signs of toxicity. All the animals are ultimately killed and autopsied.
- Acute Skin irritation/corrosion: The in vivo methods generally involve the application of the test substance to an area of an animal’s (usually an albino rabbit) skin. The animals are then observed for signs of irritation (i.e. redness and swelling) for 14 days.
- Acute eye irritation/corrosion: The test substance is applied to the conjunctival sac of one eye of each animal (usually albino rabbits). The nature and severity of the lesions and their reversibility or lack of reversibility are then observed. Topical anaesthetics and system analgesics can be used to minimise pain and distress.
- Skin sensitisation: Guinea pigs are primarily used, but recently mouse models for assessing sensitisation potential have been developed. The test animals are exposed to the test substance on their skin or via an injection within their skin. All skin reactions and any unusual findings are then observed and recorded.
OVERVIEW TABLE
WHAT CAN BE DONE ABOUT IT?
Now that we are armed with this information, we can start addressing these issues one by one!
For instance, when the Medicines Act was reviewed in the new Therapeutic Products Bill, we were able to assess the issues in the Bill. As laws are reviewed in the future, we will campaign where necessary to remove outdated requirements.
We thank you for your continued support as we make these improvements. When we need help, we will send action alerts via email updates, so make sure you’re signed up here.






